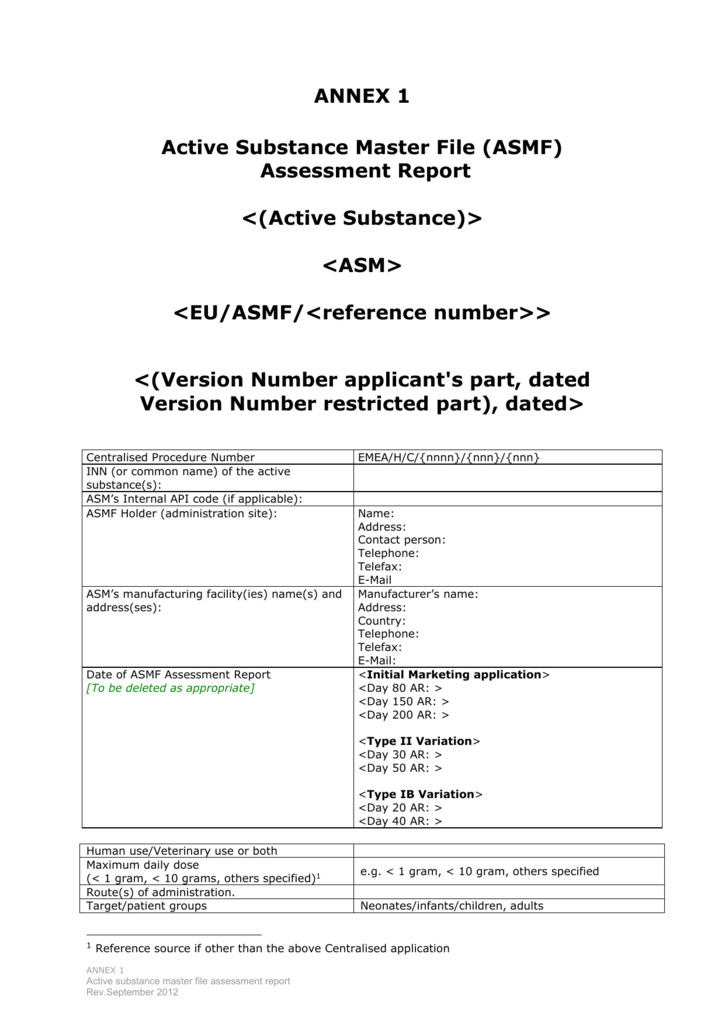
How to find a list of firms registering Active Substance Master file in Europe - Pharmaguideline Forum

Active Substance Master File Procedure PDF | PDF | Specification (Technical Standard) | Pharmaceutical

PPT - EU requirements for quality of APIs in Marketing Authorisation Application Latest developments PowerPoint Presentation - ID:6616483
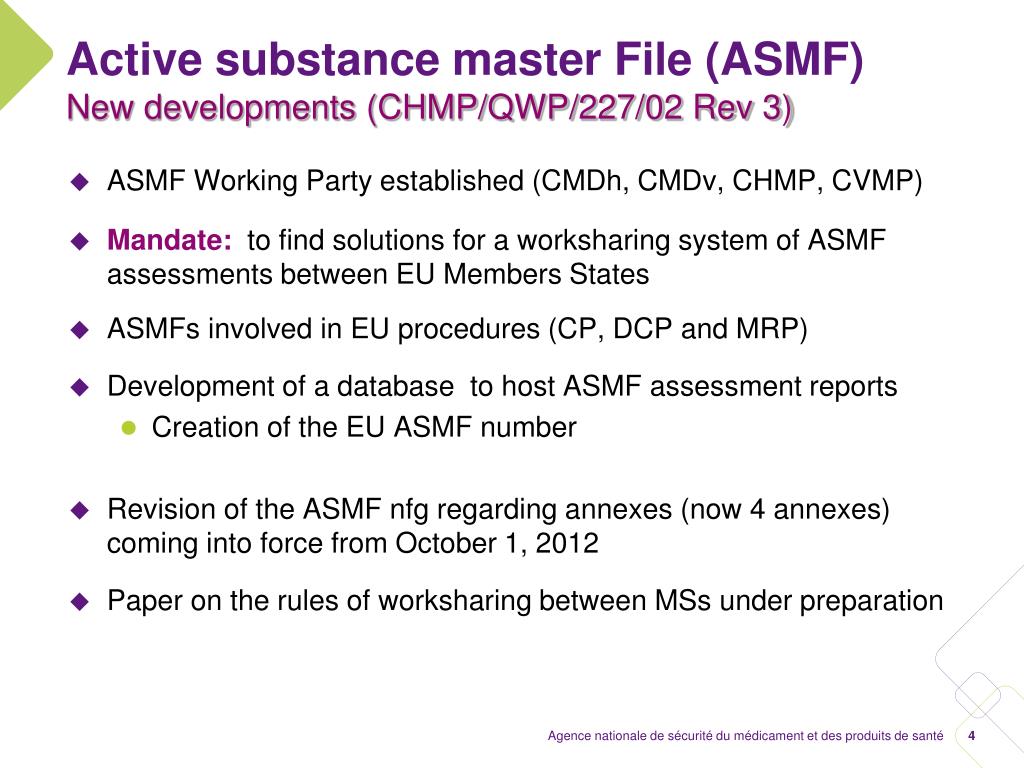
PPT - EU requirements for quality of APIs in Marketing Authorisation Application Latest developments PowerPoint Presentation - ID:6616483
![PDF] Similarities and Differences of International Practices and Procedures for the Regulation for Active Substance Master Files/Drug Master Files of Human Use: Moving Toward Regulatory Convergence. | Semantic Scholar PDF] Similarities and Differences of International Practices and Procedures for the Regulation for Active Substance Master Files/Drug Master Files of Human Use: Moving Toward Regulatory Convergence. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/9118434e508fec68995010efb453b944b1a0bdb6/3-Table1-1.png)
PDF] Similarities and Differences of International Practices and Procedures for the Regulation for Active Substance Master Files/Drug Master Files of Human Use: Moving Toward Regulatory Convergence. | Semantic Scholar